Atmospheric Ozone
Why Worry?
Stratosphere Ozone Depletion
The term "ozone depletion" means more than just the natural destruction of ozone, it means that ozone loss is exceeding ozone creation. Think again of the "leaky bucket." Putting additional ozone-destrying compounds such as CFCs into the symosphere is like causing the "bucket" of ozone to spring extra leaks. The extra leaks cause ozone to leak out at a faster rate - faster than ozone is being created. Consequently, the level of ozone protecting us from ultraviolet radiation decreases.
In the area over Antarctica stratosphere clouds hold ice particles that are not present at warmer latitudes. Reactions occur on the surface of the ice particles that accelerate the ozone destruction caused by stratospheric chlorine. This phenomenon has caused documented decreases in ozone concentrations over Antarctica. In fact, ozone levels drop so low in spring in the southern hemisphere that scientists have observed what they call a "hole" in the ozone layer.
In addition, scientists have observed declining concentrations of ozone over the whole globe. In the second half of 1992, for example, world-wide ozone levels were the lowest ever recorded.
Ozone Location
Within the major divisions of the Earth's atmosphere, the stratosphere contains a small concentration of ozone in a layer that provides Earth with a protective shield against ultraviolet (UV) radiation. After years of observation and experimentation, it seems clear that the ozone layer is affected by natural and manmade activities. And while the effects of natural events, such as volcanic eruptions, pose little threat to the equilibrium of the global Earth system, human activities are generating detrimental long-term trends which may prove to be irreversible.
Ozone is a relatively unstable molecule in Earth's atmosphere. Most ozone is concentrated below a 30-mile (48-kilometer) heigth. An ozone molecule is made up of three atoms of oxygen. Although it represents only a tiny fraction of the atmosphere, ozone is crucial for life on Earth.
Effects of Ozone
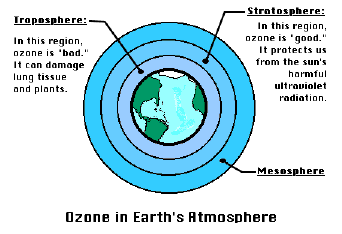
Depending on where ozone resides, it can protect or harm life on Earth. High in the atmosphere - about 15 miles (24 kilometers) up - in the stratosphere, ozone acts as a shield to protect Earth's surface from the sun's harmful ultraviolet radiation. Without this shield, we would be more susceptible to skin cancer, cataracts, and impaired immune systems. Closer to Earth in the air we breathe - Troposphere -, ozone is a harmful pollutant that causes damage to lung tissue and plants. It is also considered to be a greenhouse gas, which may contribute to global warming.
Ozone Balance
The amount of "good" and "bad" ozone in the atmosphere depends on a balance between processes that create ozone and those that destroy it. An upset in the ozone balance can have serious consequences for life on Earth. Scientists are finding evidence that changes are occurring in ozone levels - the "bad" ozone is increasing in the air we breathe, and the "good" ozone is decreasing in our protective ozone shield.
The characteristics of ozone that makes it so valuable to us - its ability to absorb a range of ultraviolet rays - also causes its destruction. When an ozone molecule (O3) absorbs even low energy ultraviolet radiation, it splits into an ordinary oxygen molecule (O2) and a free oxygen atom (O). The free oxygen atom then may join up with an oxygen molecule to make another ozone molecule, or it may steal an oxygen atom from an ozone molecule to make two ordinary oxygen molecules. Some scientists call these processes of ozone production and destruction, initiated by ultraviolet radiation, the "Chapman Reactions."
Natural forces other than the "Chapman Reactions" also affect the concentration of ozone in the stratosphere. Because ozone is a highly unstable molecule, it reacts very easily, readily donating its "extra" oxygen molecule to nitrogen, hydrogen, and chlorine found in natural compounds. These elements always have existed in the stratosphere, released from sources such as soil, water vapor, and the oceans.
In addition, scientists are finding that ozone levels change periodically as part of regular natural cycles such as the changing seasons, sun cycles and winds. Moreover, volcanic eruptions may inject materials into the stratosphere that can destroy ozone.
Over the Earth's lifetime, natural processes have regulated the balance of ozone in the stratosphere. A simple way to understand the ozone balance is to think of a leaky bucket. As long as water is poured into the bucket at the same rate that water is leaking out, the amount of water in the bucket will remain the same. Likewise, as long as ozone is being created at the same rate that it is being destroyed, the total amount of ozone will remain the same.
In the pase two decades, however, scientists have found evidence that human activities are disrupting the ozone balance. Human production of chlorine-containing chemicals such as chlorofluoro-carbons (CFCs) has added an additional force that destroys ozone. CFCs are compounds made up of chlorine, fluorine and carbon bound together. Because they are such stable molecules, CFCs do not react easily with other chemicals in the lower atmosphere. One of the few forces that can break up CFC molecules is ultraviolet radiation. In the lower atmosphere, however, CFCs are protected from ultraviolet radiation by the ozone layer. CFC molecules thus are able to migrate intact up into the stratosphere. Although the CFC molecules are heavier than air, the mixing processes of the atmosphere carry them into the stratosphere.
Once in the stratosphere, however, the CFC molecules no longer are shielded from ultraviolet radiation by the ozone layer. Bombarded by the sun's ultraviolet energy, CFC molecules break up and release their chlorine atoms. The free chlorine atoms then can react with ozone molecles, taking one oxygen atom to form chlorine monoxide and leaving an ordinary oxygen molecule.
If each chlorine atom released from a CFC molecule destroyed only one ozone molecule, CFCs probably would pose very little threat to the ozone layer. However, when a chlorine monoxide molecule encounters a free atom of oxygen, the oxygen atom breaks up the chlorine monoxide, stealing the oxygen atom and releasing the chlorine atom back into the stratosphere to destroy more ozone. This reactionhappens over and over again, allowing a single atom of chlorine to destroy many molecules of ozone.
Fortunately, chlorine atoms do not remain in the stratosphere forever. When a free chlorine atom reacts with gases such as methane (CH4), it is bound up into a molecule of hydrogen chloride (HCL), which can be carried from the stratosphere into the troposphere, where it can be washed away by rain. Therefore, if humans stop putting CFCs and other ozone-destroying chemicals into the stratosphere, the ozone layer eventually may repair itself.